Abstract
Introduction: Approximately 77% of younger pts and 49% of older pts with newly diagnosed AML achieve CR after induction chemotherapy (chemo). The majority of pts who achieve CR eventually relapse, with only approximately 30% of pts maintaining CR for 3 yrs or longer. Long-term outcomes in pts maintaining 1st CR for at least 3 yrs (AML survivors) remain largely unknown. The purpose of this study was to investigate long-term outcomes in this subset of pts.
Methods: We performed a chart review of pts with AML treated at our institution from 2000-2015 who achieved CR for at least 3 yrs after their initial chemo ± allogeneic stem cell transplant (ASCT) to analyze their long-term outcomes.
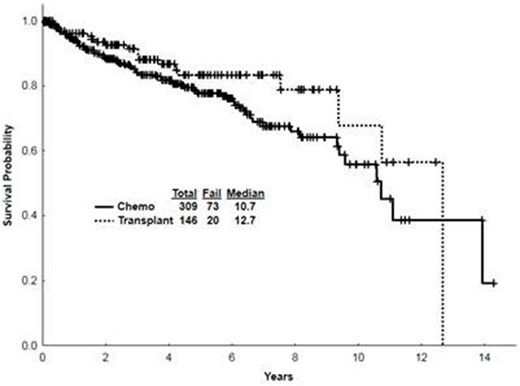
Results: 2779 pts with AML were treated between 2000 and 2015 at our institution with 1686 (61%) pts achieving CR. Among them, 455 (27%; 16% of all treated) maintained 1st CR for at least 3 years and constitute the focus of this analysis. Among them 309 received chemo alone and 146 chemo followed by ASCT. The median time from the initiation of induction chemo to the first CR was 29 days [10-207] for the entire cohort, and it was similar for pts treated with chemo only versus those who later received ASCT. Baseline characteristics for AML survivors were as follows: hypertension (HTN) in 27% of pts; dyslipidemia (DLD) in 16%; pulmonary disease in 10%; cardiac disease in 9%; diabetes mellitus (DM) in 9%; depression in 5%, renal disease in 5%; gastroesophageal reflux disease (GERD) in 5%; hypothyroidism in 5%; anxiety in 4%; osteopenia/osteoporosis in 1%. Late relapses (i.e., after 3 years in CR) occurred in 11% of pts - 14% treated with chemo alone and 6% treated with ASCT. Such relapses occurred after a median CR duration of 6 yrs (3-12) and 7 yrs (4-12) respectively. A change in karyotype compared to the original karyotype was seen in 51% of relapsed chemo pts and 56% of relapsed ASCT pts. The most common new cytogenetic abnormalities were 7q del (22%), Trisomy 8 (11%), and 5q del (7%). The two most common mutational status changes seen in relapsed pts were FLT3-ITD appearing in 5% of chemo pts while disappearing in 11% of ASCT pts and FLT3-D835 appearing in 5% of chemo pts while disappearing in 2% of chemo pts. Patients treated with ASCT did not display a change in FLT3-D835. At relapse, TP53 mutation was seen in 7% of pts; however, TP53 status at the time of diagnosis was unknown. A 2nd complete remission (CR2) was achieved in 54% of late relapse pts: 51% of chemo only pts and 67% of ASCT pts. The median CR2 duration was 18 months (mo) [3-127] and 15 [7-23] respectively. The median survival after relapse was 10 mo in chemo only pts and 14 mo in ASCT pts. Of chemo pts who relapsed, 30% underwent an ASCT and 4 ASCT pts received a 2nd ASCT. New comorbidities that were present at or after the 3 yr mark included: HTN in 15%; osteopenia/osteoporosis in 15%; renal disease in 14%; pulmonary disease in 11%; hematologic complications in 11%; DLD in 9%; GERD in 8%; cardiac ailments in 7%; depression and anxiety in 7% and 6% respectively; DM in 6%; hypothyroidism in 4%. Osteopenia/osteoporosis was seen in 39% of ASCT pts and 3% of chemo pts. Renal disease was seen in 23% of ASCT pts and 10% of chemo pts. Pulmonary disease was seen in 16% of ASCT pts and 9% of chemo pts. Second malignancies occurred in 17% of pts with the most common being skin cancer (7%), prostate cancer (2%), breast cancer (1%), and lymphoma (1%). Skin cancer occurred in 12% of ASCT pts and 5% of chemo pts. The median survival for the entire cohort starting from 3 yrs in CR is 10.7 yrs (0.1-14.3) with chemo pts having a median survival of 10.7 yrs (0.1-14.3) and 12.7 yrs (0.1-12.7) for ASCT pts (Fig 1). The most common causes of death (COD) for relapse ASCT pts was relapsed AML (33%), complications secondary to 2nd ASCT (22%), and infection (11%); the most common COD for relapse chemo pts was relapsed AML (47%), complications secondary to ASCT (14%), and second malignancy (2%). The most common COD for CR1 ASCT pts was ASCT-related complications (2%), malignancy (<1%), and myocardial infarction (MI) (<1%). The most common COD for CR1 chemo was malignancy (2%), MI (<1%), and pneumonia (<1%).
Conclusion: The incidence of relapse among AML pts who have maintained CR for at least three yrs is low. However, new medical problems occur frequently, including HTN, osteoporosis, and second malignancies - with skin cancer being especially prevalent among ASCT recipients. Long-term survivors of AML require routine surveillance as well as preventative measures.
Kadia:Novartis: Consultancy; BMS: Research Funding; Celgene: Research Funding; Pfizer: Consultancy, Research Funding; Celgene: Research Funding; Abbvie: Consultancy; BMS: Research Funding; Takeda: Consultancy; Novartis: Consultancy; Pfizer: Consultancy, Research Funding; Abbvie: Consultancy; Jazz: Consultancy, Research Funding; Jazz: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Takeda: Consultancy; Amgen: Consultancy, Research Funding. Pemmaraju:celgene: Consultancy, Honoraria; abbvie: Research Funding; cellectis: Research Funding; stemline: Consultancy, Honoraria, Research Funding; samus: Research Funding; daiichi sankyo: Research Funding; SagerStrong Foundation: Research Funding; plexxikon: Research Funding; novartis: Research Funding; Affymetrix: Research Funding. Short:Takeda Oncology: Consultancy. Daver:ImmunoGen: Consultancy; Alexion: Consultancy; Karyopharm: Consultancy; Incyte: Consultancy; Sunesis: Consultancy; Daiichi-Sankyo: Research Funding; Sunesis: Research Funding; Incyte: Research Funding; Pfizer: Research Funding; Karyopharm: Research Funding; Novartis: Research Funding; Pfizer: Consultancy; ARIAD: Research Funding; Otsuka: Consultancy; Novartis: Consultancy; Kiromic: Research Funding; BMS: Research Funding. Jabbour:novartis: Research Funding. Champlin:Otsuka: Research Funding; Sanofi: Research Funding. Konopleva:cellectis: Research Funding; abbvie: Research Funding; Stemline Therapeutics: Research Funding; Immunogen: Research Funding. Andreeff:Celgene: Consultancy; Jazz Pharma: Consultancy; Reata: Equity Ownership; Eutropics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Patents & Royalties: MDM2 inhibitor activity patent, Research Funding; Oncolyze: Equity Ownership; United Therapeutics: Patents & Royalties: GD2 inhibition in breast cancer ; SentiBio: Equity Ownership; Astra Zeneca: Research Funding; Aptose: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Research Funding; Oncoceutics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Wierda:AbbVie, Inc: Research Funding; Genentech: Research Funding. Ravandi:Macrogenix: Honoraria, Research Funding; Abbvie: Research Funding; Astellas Pharmaceuticals: Consultancy, Honoraria; Amgen: Honoraria, Research Funding, Speakers Bureau; Sunesis: Honoraria; Seattle Genetics: Research Funding; Bristol-Myers Squibb: Research Funding; Abbvie: Research Funding; Seattle Genetics: Research Funding; Orsenix: Honoraria; Jazz: Honoraria; Sunesis: Honoraria; Xencor: Research Funding; Astellas Pharmaceuticals: Consultancy, Honoraria; Amgen: Honoraria, Research Funding, Speakers Bureau; Macrogenix: Honoraria, Research Funding; Xencor: Research Funding; Jazz: Honoraria; Bristol-Myers Squibb: Research Funding; Orsenix: Honoraria. Cortes:novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal